relativistic mass is the mass change with speed. RELATIVISTIC CONTRACTION
The Lorentz contraction is a relativistic effect is the contraction of the size of a body as its speed is comparable to speeds approaching the speed of light. Originally a concept introduced by Lorentz as a way to explain the absence of positive results in the Michelson-Morley experiment. It was subsequently deduced by Albert Einstein in the context of special relativity.
The Lorentz contraction is described by the following expression

where L0 is the distance measured by a stationary observer and L1 is the distance measured by an observer moving at a speed v where c is the speed of light.
Lorentz contraction can also be understood as the effect of time dilation as the increase of inertial mass of a body or particle.
Time Dilation Time dilation is the phenomenon predicted by the theory of relativity, by which an observer notes that the other clock (a clock physically identical to yours) is marking time at a lower rate measuring the clock. This is usually interpreted usually as the weather has slowed for the other clock, but this is true only in the context of the reference system of the observer. Locally, the time is always happening at the same pace. The phenomenon of time dilation applies to any process that manifests changes over time.
Einstein POSTULATES
1 .- The laws of physics are valid and have the same mathematical expression in all inertial reference systems.
2. The speed of light is the same for all inertial systems. In other words, the speed of light is the same whatever the focus movement and the observer.
CONSEQUENCES OF THE THEORY OF RELATIVITY
1. The laws of motion Newton is a real approximation of other more general laws.
2. The notion of space, taken in isolation, is meaningless, it just really the whole spacetime.
3. No velocity can exceed the speed of light.
4. 4. Energy is endowed with a kind of inertia and is equivalent to matter
5. The body dimensions vary with the speed of which are animated. Thus, a sphere is transformed into an ellipsoid flattened in the direction of movement. When the body reaches the speed of light, the third dimension disappears to the point of appearing the body without thickness.
6. The mass of a moving body increases speed, until it becomes infinite if the body reaches the speed of light.
7. There is no general time. And all, but local time specific to each reference system.
PLANCK POSTULATE
Planck's constant, symbolized by the letter h (or h = h/2π, in which case they are called reduced Planck constant), is a physical constant that represents the elementary quantum of action . Is the relationship between the amount of energy and often associated with a quantum or a particle. Plays a central role in the theory of quantum mechanics and is named after its discoverer, Max Planck, one of the fathers of the theory.
Planck's constant relates the energy E of the photons with frequency ν of the light wavelength (Greek letter nu) using the formula:
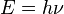
JOB FUNCTION
The work function is the minimum energy ( usually measured in electron volts) needed to remove an electron from a solid to a point immediately outside the solid surface (or the energy required to move an electron from the Fermi energy level to vacuum.) Here "immediately" means that the final position of the electron is far from the surface at the atomic scale but still close to the solid on a macroscopic scale. The work function is a fundamental property for any solid substance condución a banda (both empty and partially full.) For a metal, the Fermi level is inside the conduction band, indicating that the band is partially filled. For an insulator, the Fermi level falls within the gap, indicating an empty conduction band, in this case, the minimum energy to remove an electron is approximately the sum of half the gap, and job function. PHOTOELECTRIC EFFECT
The photoelectric effect is the emission of electrons from a material when illuminated by radiation electromagnetic (visible or ultraviolet light in general). Sometimes the term is included in other types of interaction between light and matter:
* photoconductivity: the increase in electrical conductivity of the material or caused by light diodes. Discovered by Willoughby Smith in selenium in the mid-nineteenth century.
* Photovoltaic effect: partial conversion of light energy into electrical energy. The first solar cell was built by Charles Fritts in 1884. It consisted of selenium coated with a thin layer of gold.
The photoelectric effect was discovered and described by Heinrich Hertz in 1887. The theoretical explanation was only made by Albert Einstein in 1905 who based his formulation of the photoelectric work on an extension of the Max Planck quanta. Robert Andrews Millikan later spent ten years experimenting to prove Einstein's theory was not correct ... and showed that it was. That allowed Einstein and Millikan shared the Nobel prize in 1921 and 1923 respectively.

diagram of the photoelectric effect. The incident photons are absorbed by the electrons of the medium providing them with enough energy to escape from it. SPECTROMETER
The spectrometer is an apparatus capable of analyzing the spectrum characteristic of a wave motion. It applies to various instruments that operate on a wide range of wavelengths.
EMISSION LINE SPECTRUM AND ABSORPTION
Each atom can emit or absorb electromagnetic radiation, but only at certain frequencies that are characteristic of each of the different chemical elements.
If, through the provision of heat energy, it stimulates a particular element in its gaseous phase, the atoms emit radiation at certain frequencies of the visible, which constitute its emission spectrum.
If the same element, also in a state of gas, receives radiation electromagnetic absorbs certain frequencies of visible, precisely those in which emits when stimulated by heat. This is its absorption spectrum.
Met and the so-called Kirchoff's Law, which tells us that every element absorbs radiation at the same wavelengths at which the issue. The absorption spectra and emission therefore appears to be the negative of each other. SPECTRAL SERIES
The spectral range is explained by the crystal field model that is related to the colors seen in different complexes, and this is so because the difference in energy between orbitals is the same order of magnitude energy of a photon of visible light, which excites an electron from the orbitals of lower energy towards higher energy. The magnitude of the difference in energy and therefore the color of a complex depends on both the metal and the ligands that surround it.
Bohr atom model of Bohr and Bohr-Rutherford is a quantized model of the atom that Bohr proposed in 1913 to explain how electrons can have stable orbits around the nucleus. This planetary model is a functional model that represents the atom (physical object) itself but explains how it works through equations.
Niels Bohr atom was based on hydrogen for the model that bears his name. Bohr tried to make an atomic model can explain the stability of matter and the emission and absorption spectra observed in discrete gases. He described the hydrogen atom with one proton in its nucleus, and revolving around an electron. Bohr's atomic model was based conceptually atomic model of Rutherford and emerging ideas about quantization that had arisen a few years earlier with the investigations of Max Planck and Albert Einstein. Because of its simplicity, the Bohr model is still often used as a simplification of the structure of matter.
 Diagram
Diagram Bohr atom.
principal quantum number quantum numbers describe the values \u200b\u200bof dynamic variables that are stored in quantum systems. Therefore correspond to those observables that commute with the Hamiltonian of the system. Thus, the quantum numbers can characterize the stationary states, ie eigenstates of the Hamiltonian.
In atomic physics, quantum numbers are discrete numerical values \u200b\u200bthat indicate the characteristics of electrons in atoms, this is based on the atomic theory of Niels Bohr's atomic model more accepted and used in recent times for its simplicity.
In particle physics the term is also used to designate the quantum numbers of possible values \u200b\u200bof certain observable or physical quantity possessing a spectrum or range of possible discrete values.
ENERGY LEVEL
energy levels are the electrons spinning around the nucleus forming layers. In each, the energy possessed by the electron is different. Indeed, in the layers very close to the nucleus, the attractive force between it and the electron is very strong, so it will be strongly linked. The
made therefore that the electrons in an atom have different energy levels, leads us to classify the energy level (or energy band) that meets every one of them. Bands that interests us to better understand the behavior of the atom are:
- La Banda de Valencia: The energy level in which chemical compounds are made. The electrons in it, can be transferred from one atom to another, forming ions that attract due to their different charge, or will be shared by several atoms, forming molecules.
- La Banda driving: it is an energy level in which electrons are further isolated from the core, so that, in a sense, all the electrons (belonging to the band) are shared by all atoms of the solid, and can scroll through this forming an electron cloud.